KazNU scientists have developed a new method of splitting toxic substances

Scientists at Al-Farabi Kazakh National University have developed an innovative method of splitting organic and inorganic toxic substances at the molecular level using photocells.
The project "Nanoscale film structures based on widely forbidden metal oxides and plasmonic nanoparticles with specified electronic properties" is realized under the guidance of PhD Associate Professor Erzhan Mukhametkarimov.

Today, one of the urgent problems is the fight against pollution of the environment with organic and inorganic toxic substances, so the project is based on the preservation of terrestrial ecosystems in line with the realization of sustainable development goals. One of the ways to combat pollution is to break it down at the molecular level with the help of photovoltaic materials. The most effective photovoltaic materials are conductors with a wide bandgap, such as ZnO and TiO2. These materials are cheap and low-toxic, have stable chemical composition and are important for practical applications. The energy of charge carriers in these materials is generated by radiation. And these redox reactions are sufficient to destroy adsorbed contaminating macromolecules on the surface of the material.

The main problem restraining their large-scale application is that due to the large width of the forbidden zone (over 3 eV), the generation of charge carriers occurs only under the influence of UV radiation, which is only 4% of the entire solar spectrum, as well as a high degree of their recombination.
Therefore, this project investigates the possibility of increasing the photoactivity of TiO2 and ZnO thin films by creating their composition with nanoparticles of noble metals: silver, gold and their alloys. Metal nanoparticles improve the functional characteristics of TiO2 and ZnO thin films in the visible range.
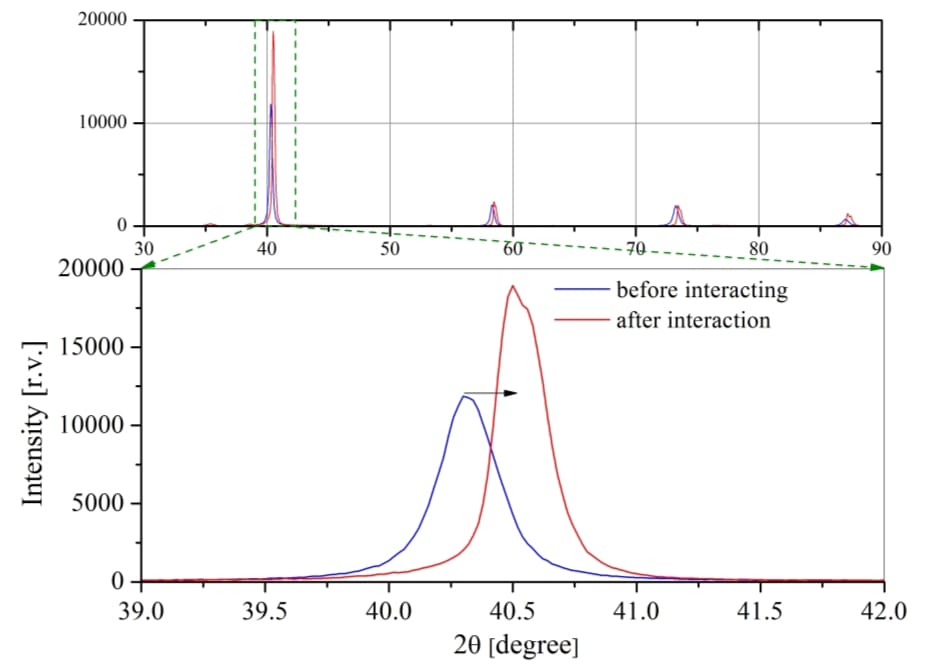
Photoactivity is assessed by various methods such as: analyzing the rate of dye degradation under UV and visible light, photoelectrochemical measurements using electrochemical cells.
Press- Service of Al- Farabi Kazakh National University.
